Hashimoto's thyroiditis
| Hashimoto's thyroiditis | |
|---|---|
| Other names | Chronic lymphocytic thyroiditis, autoimmune thyroiditis, struma lymphomatosa, Hashimoto's disease |
 | |
| A micrograph of the thyroid of someone with Hashimoto's thyroiditis | |
| Specialty | Endocrinology |
| Symptoms | Painless goiter, weight gain, feeling tired, constipation, depression, dry skin, hair loss[1] |
| Complications | Thyroid lymphoma.[2] |
| Usual onset | 30–50 years old[1][3] |
| Causes | Genetic and environmental factors.[4] |
| Risk factors | Family history, another autoimmune disease[1] |
| Diagnostic method | TSH, T4, anti-thyroid autoantibodies, ultrasound[1] |
| Differential diagnosis | Graves' disease, nontoxic nodular goiter[5] |
| Treatment | Levothyroxine, surgery[1][5] |
| Frequency | 2% at some point[4] |
Hashimoto's thyroiditis, also known as chronic lymphocytic thyroiditis, Hashimoto's disease, and autoimmune thyroiditis is an autoimmune disease in which the thyroid gland is gradually destroyed.[6][7]
Early on, symptoms may not be noticed.[1] Over time, the thyroid may enlarge, forming a painless goiter.[1] Some people eventually develop hypothyroidism with accompanying weight gain, fatigue, constipation, depression, hair loss, and general pains.[1] After many years the thyroid typically shrinks in size.[1] Potential complications include thyroid lymphoma.[2] Furthermore, because it is common for untreated patients of Hashimoto's to develop hypothyroidism, further complications can include, but are not limited to, high cholesterol, heart disease, heart failure, high blood pressure, myxedema, and potential pregnancy problems.[7]
Hashimoto's thyroiditis is thought to be due to a combination of genetic and environmental factors.[4] Risk factors include a family history of the condition and having another autoimmune disease.[1] Diagnosis is confirmed with blood tests for TSH, T4, antithyroid autoantibodies, and/or ultrasound.[1] Other conditions that can produce similar symptoms include Graves' disease and nontoxic nodular goiter.[5]
Hashimoto's thyroiditis is typically treated with levothyroxine.[1][8] If hypothyroidism is not present, some may recommend no treatment, while others may treat to try to reduce the size of the goiter.[1][9] Those affected should avoid eating large amounts of iodine; however, sufficient iodine is required especially during pregnancy.[1] Surgery is rarely required to treat the goiter.[5]
Hashimoto's thyroiditis affects about 5% of white people of Western European descent at some point in their lives.[4] It is the most common cause of hypothyroidism in iodine-sufficient areas of the world.[10] It typically begins between the ages of 30 and 50 and is much more common in women than men.[1][3] Rates of the disease appear to be increasing.[5] It was first described by the Japanese physician Hakaru Hashimoto in 1912.[11] In 1957, it was recognized as an autoimmune disorder.[12]
Signs and symptoms
[edit]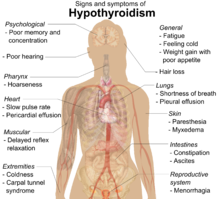
Signs
[edit]
Early stages of autoimmune thyroiditis may have a normal physical exam with or without a goiter.[13] A goiter is a diffuse, often symmetric, swelling of the thyroid gland visible in the anterior neck that may develop.[13] The thyroid gland may become firm, large, and lobulated in Hashimoto's thyroiditis, but changes in the thyroid can also be nonpalpable.[14] Enlargement of the thyroid is due to lymphocytic infiltration[15] and fibrosis, rather than tissue hypertrophy.
While their role in the initial destruction of the follicles is unclear, antibodies against thyroid peroxidase or thyroglobulin are relevant, as they serve as markers for detecting the disease and its severity.[16] They are thought to be the secondary products of the T cell-mediated destruction of the gland.[17]
As lymphocytic infiltration progresses, patients may exhibit signs of hypothyroidism in multiple bodily systems, including, but not limited to, a larger goiter, weight gain, cold intolerance, fatigue, myxedema, constipation, menstrual disturbances, pale or dry skin, and dry, brittle hair, depression, and ataxia.[13][10] Extended thyroid hormone deficiency may lead to muscle fibre changes, with fast-twitching type II being replaced by slow-twitching type-I fibers, resulting in muscle weakness, muscle pain, stiffness, and rarely, pseudohypertrophy[18].
While rare, more serious complications of the hypothyroidism resulting from autoimmune thyroiditis are pericardial effusion, pleural effusion, both of which require further medical attention, and myxedema coma, which is an endocrine emergency.[10]
Patients with goiters who have had autoimmune thyroiditis for many years might see their goiter shrink in the later stages of the disease due to destruction of the thyroid.[19]
Graves disease may occur before or after the development of autoimmune thyroiditis.[20]
Symptoms
[edit]Many symptoms are attributed to the development of Hashimoto's thyroiditis. The most common symptoms include: fatigue, weight gain, pale or puffy face, feeling cold, joint and muscle pain, constipation, dry and thinning hair, heavy menstrual flow or irregular periods, depression, panic disorder, a slowed heart rate, problems getting pregnant, miscarriages[21], and myopathy[22].
Some patients in the early stage of the disease may experience symptoms of hyperthyroidism due to the release of thyroid hormones from intermittent thyroid destruction.[19][23]
While most symptoms are attributed to hypothyroidism, several studies have observed symptoms in hashimotos patients with normal thyroid hormone levels.[24][25] These symptoms may include lower quality of life, "digestive system (abdominal distension, constipation and diarrhea), endocrine system (chilliness, gain weight and facial edema), neuropsychiatric system (forgetfulness, anxiety, depressed, fatigue, insomnia, irritability, and indifferent) and mucocutaneous system (dry skin, pruritus, and hair loss)."[26]
Causes
[edit]The causes of Hashimoto's thyroiditis are complex. Around 80% of the risk of developing an autoimmune thyroid disorder is due to genetic factors, while the remaining 20% is related to environmental factors (such as iodine, drugs, infection, stress, radiation).[27]
Genetics
[edit]Thyroid autoimmunity can be familial.[28] Many patients report a family history of autoimmune thyroiditis or Graves' disease.[13] The strong genetic component is borne out in studies on monozygotic twins[10], with a concordance of 38–55%, with an even higher concordance of circulating thyroid antibodies not in relation to clinical presentation (up to 80% in monozygotic twins). Neither result was seen to a similar degree in dizygotic twins, offering strong favour for high genetic aetiology.[29]
The genes implicated vary in different ethnic groups[30] and the impact of these genes on the disease differs significantly among people from different ethnic groups. A gene that has a large effect in one ethnic group's risk of developing hashimoto's thyroiditis might have a much smaller effect in another ethnic group.[29]
The incidence of autoimmune thyroid disorders is increased in people with chromosomal disorders, including Turner, Down, and Klinefelter syndromes.[27]
HLA genes
[edit]The first gene locus associated with autoimmune thyroid disease was the major histocompatibility complex (MHC) region on chromosome 6p21. It encodes human leukocyte antigens (HLAs). Specific HLA alleles have a higher affinity to autoantigenic thyroidal peptides and can contribute to autoimmune thyroid disease development. Specifically, in Hashimoto's disease, aberrant expression of HLA II on thyrocytes has been demonstrated. They can present thyroid autoantigens and initiate autoimmune thyroid disease.[30] Susceptibility alleles are not consistent in Hashimoto's disease. In Caucasians, various alleles are reported to be associated with the disease, including DR3, DR5, and DQ7.[31][32]
CTLA-4 genes
[edit]CTLA-4 is the second major immune-regulatory gene related to autoimmune thyroid disease. CTLA-4 gene polymorphisms may contribute to the reduced inhibition of T-cell proliferation and increase susceptibility to autoimmune response.[33] CTLA-4 is a major thyroid autoantibody susceptibility gene. A linkage of the CTLA-4 region to the presence of thyroid autoantibodies was demonstrated by a whole-genome linkage analysis.[34] CTLA-4 was confirmed as the main locus for thyroid autoantibodies.[35]
PTPN22 gene
[edit]PTPN22 is the most recently identified immune-regulatory gene associated with autoimmune thyroid disease. It is located on chromosome 1p13 and expressed in lymphocytes. It acts as a negative regulator of T-cell activation. Mutation in this gene is a risk factor for many autoimmune diseases. Weaker T-cell signaling may lead to impaired thymic deletion of autoreactive T cells, and increased PTPN22 function may result in inhibition of regulatory T cells, which protect against autoimmunity.[36]
Immune-related genes
[edit]IFN-γ promotes cell-mediated cytotoxicity against thyroid mutations causing increased production of IFN-γ were associated with the severity of hypothyroidism.[37] Severe hypothyroidism is associated with mutations leading to lower production of IL-4 (Th2 cytokine suppressing cell-mediated autoimmunity),[38] lower secretion of TGF-β (inhibitor of cytokine production),[39] and mutations of FOXP3, an essential regulatory factor for the regulatory T cells (Tregs) development.[40] Development of Hashimoto's disease was associated with mutation of the gene for TNF-α (stimulator of the IFN-γ production), causing its higher concentration.[41]
Sex
[edit]Study of healthy Danish twins divided to three groups (monozygotic and dizygotic same sex, and opposite sex twin pairs) estimated that genetic contribution to thyroid peroxidase antibodies susceptibility was 61% in males and 72% in females, and contribution to thyroglobulin antibodies susceptibility was 39% in males and 75% in females.[42]
The high female predominance in thyroid autoimmunity may be associated with the X chromosome. It contains sex and immune-related genes responsible for immune tolerance.[43] A higher incidence of thyroid autoimmunity was reported in patients with a higher rate of X-chromosome monosomy in peripheral white blood cells.[44] Another potential mechanism might be skewed X-chromosome inactivation, leading to the escape of X-linked self-antigens from presentation in the thymus and loss of T-cell tolerance.[citation needed]
Environmental
[edit]Medications
[edit]Certain medications or drugs have been associated with altering and interfering with thyroid function. Of these drugs, there are two main mechanisms of interference that they can have.[45]
One of the mechanisms of interference is when a drug alters thyroid hormone serum transfer proteins.[45] Estrogen, tamoxifen, heroin, methadone, clofibrate, 5-flurouracile, mitotane, and perphenazine all increase thyroid binding globulin (TBG) concentration.[45] Androgens, anabolic steroids such as danazol, glucocorticoids, and slow release nicotinic acid all decrease TBG concentrations. Furosemide, fenoflenac, mefenamic acid, salicylates, phenytoin, diazepam, sulphonylureas, free fatty acids, and heparin all interfere with thyroid hormone binding to TBG and/or transthyretin.[45]
The other mechanism that medications can utilize to interfere with thyroid function would be to alter extra-thryoidal metabolism of thyroid hormone. Propylthiouracil, glucocorticoids, propranolol, iondinated contrast agents, amiodarone, and clomipramine all inhibit conversion of T4 and T3.[45] Phenobarbital, rifampin, phenytoin and carbamazepine all increase hepatic metabolism.[45] Finally, cholestryamine, colestipol, aluminium hydroxide, ferrous sulphate, and sucralfate are all drugs that decrease T4 absorption or enhance excretion.[45]
Iodine
[edit]Excessive iodine intake is a well-established environmental factor for triggering thyroid autoimmunity. Thyroid autoantibodies are found to be more prevalent in geographical areas with a higher dietary iodine levels. Several mechanisms by which iodine may promote thyroid autoimmunity have been proposed. Iodine exposure leads to higher iodination of thyroglobulin, increasing its immunogenicity by creating new iodine-containing epitopes or exposing cryptic epitopes. It may facilitate presentation by APC, enhance the binding affinity of the T-cell receptor, and activate specific T-cells.[46]
Iodine exposure has been shown to increase the level of reactive oxygen species. They enhance the expression of the intracellular adhesion molecule-1 on the thyroid follicular cells, which could attract the immunocompetent cells into the thyroid gland.[47]
Iodine is toxic to thyrocytes since highly reactive oxygen species may bind to membrane lipids and proteins. It causes thyrocyte damage and the release of autoantigens. Iodine also promotes follicular cell apoptosis and has an influence on immune cells (augmented maturation of dendritic cells, increased number of T cells, stimulated B-cell immunoglobulin production).[48][49]
Data from the Danish Investigation of Iodine Intake and Thyroid Disease shows that within two cohorts (males, females) with moderate and mild iodine deficiency, the levels of both thyroid peroxidase and thyroglobulin antibodies are higher in females, and prevalence rates of both antibodies increase with age.[50]
Pregnancy
[edit]Two or more births are a risk factor for developing autoimmune hypothyroidism in pre-menopausal women.[51]
Comorbidities
[edit]Comorbid autoimmune diseases are a risk factor for developing Hashimoto's thyroiditis, and the opposite is also true.[1] Another thyroid disease closely associated with Hashimoto's thyroiditis is Graves' disease.[20] Autoimmune diseases affecting other organs most commonly associated with Hashimoto's thyroiditis include celiac disease, type 1 diabetes, vitiligo, alopecia,[52] Addison disease, Sjogren's syndrome, and rheumatoid arthritis.[13][19] Autoimmune thyroiditis has also been seen in patients with autoimmune polyendocrine syndromes type 1 and 2.[20]
Other
[edit]Other environmental factors including selenium deficiency, infectious diseases(e.g., hepatitis C virus, rubella virus)[53][54], toxins, dietary factors[20], gut microbiome, and radiation exposure[54] have been implicated in the development of autoimmune thyroid disease in genetically predisposed individuals.
Mechanism
[edit]The pathophysiology of autoimmune thyroiditis is not well understood, but is thought to develop as a result of a complex interaction of genetics and environmental factors.[17]
Hashimoto's Thyroiditis is a T-lymphocyte mediated attack on the thyroid gland[15]. "Th1 cells activate macrophages and cytotoxic lymphocytes which directly destroy thyroid follicular cells" and "Th2 cells lead to an excessive stimulation and production of B cells and plasmatic cells, which produce antibodies against the thyroid antigens, leading to thyroiditis."[55] Lymphocytes produce antibodies targeting three different thyroid proteins: Thyroid peroxidase Antibodies (TPOAb), Thyroglobulin Antibodies (TgAb), and Thyroid stimulating hormone receptor Antibodies (TRAb).[28][56] The two antibodies most commonly implicated in autoimmune thyroiditis are antibodies against thyroid peroxidase (TPOAb) and thyroglobulin (TgAb).[17] They are hypothesized to develop as a result of thyroid damage, where T-lymphocytes are sensitized to residual thyroid peroxidase and thyroglobulin, rather than as the cause of thyroid damage.[17] However, they may exacerbate further thyroid destruction by binding the complement system and triggering apoptosis of thyroid cells.[17]
Gross morphological changes within the thyroid are seen in the general enlargement, which is far more locally nodular and irregular than more diffuse patterns (such as that of hyperthyroidism). While the capsule is intact and the gland itself is still distinct from surrounding tissue, microscopic examination can provide a more revealing indication of the level of damage.[57]
Hypothyroidism is caused by replacement of follicular cells with parenchymatous tissue.[55]
Pathology
[edit]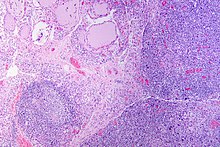

Gross pathology of a thyroid with autoimmune thyroiditis may show an symmetrically enlarged thyroid.[17] It is often paler in color, in comparison to normal thyroid tissue which is reddish-brown.[17] Microscopic examination will show infiltration of lymphocytes and plasma cells. The lymphocytes are predominately T-lymphocytes with a representation of both CD4 positive and CD8 positive cells.[17] The plasma cells are polyclonal, with present germinal centers resembling the structure of a lymph node.[17] Fibrous tissue may be found throughout the affected thyroid as well.[17] Generally, pathological findings of the thyroid are related to the amount of existing thyroid function - the more infiltration and fibrosis, the less likely a patient will have normal thyroid function.[17] In late stages of the disease, the thyroid may be atrophic.[10]
Histologically, the hypersensitivity is seen as diffuse parenchymal infiltration by lymphocytes, particularly plasma B-cells, which can often be seen as secondary lymphoid follicles (germinal centers, not to be confused with the normally present colloid-filled follicles that constitute the thyroid). Atrophy of the colloid bodies is lined by Hürthle cells, cells with intensely eosinophilic, granular cytoplasm, a metaplasia from the normal cuboidal cells that constitute the lining of the thyroid follicles. Severe thyroid atrophy presents often with denser fibrotic bands of collagen that remains within the confines of the thyroid capsule.[57]
A rare but serious complication is thyroid lymphoma, generally the B-cell type, non-Hodgkin lymphoma.[58]
Diagnosis
[edit]
Some or all of the following tests may be performed, in any order:
Physical exam
[edit]Physicians will often start by assessing reported symptoms and performing a thorough physical exam, including a neck exam.[10] On gross examination, a hard goiter that is not painful to the touch often presents;[57] other symptoms seen with hypothyroidism, such as periorbital myxedema, depend on the current state of progression of the response, especially given the usually gradual development of clinically relevant hypothyroidism.
To detect if the pituitary is inciting an underperforming thyroid to produce more thyroid hormone. TSH secretion from the anterior pituitary increases in response to decreased serum thyroid hormones. If elevated, it signifies hypothyroidism.[19] The elevation is usually a marked increase over the normal range and is generally greater than 20 mg/dl.[13]
Antithyroid antibodies tests
[edit]Tests for antibodies against thyroid peroxidase, thyroglobulin, and thyrotropin receptors, can detect autoimmune processes against the thyroid. However, seronegative (without circulating autoantibodies) thyroiditis is also possible.[59] There may be circulating antibodies before the onset any symptoms.[10]
T3 or T4 levels test
[edit]To detect a lack of thyroid hormones (ie hypothyroidism), or excess of thyroid hormones (hyperthyroidism). Typically, T4 is the preferred thyroid hormone test for hypothyroidism.[60] Free T4 levels will usually be lowered, but sometimes might be normal.[61]
Ultrasound
[edit]
An ultrasound may be useful in detecting Hashimoto thyroiditis, especially in those with seronegative thyroiditis, due to key features detected in the ultrasound of a person with Hashimoto's thyroiditis, such as "echogenicity, heterogeneity, hypervascularity, and presence of small cysts."[15]
When patients have normal laboratory values but symptoms of autoimmune thyroiditis, ultrasound plays a role in diagnosis.[19] Images obtained with ultrasound can evaluate the size of the thyroid and further support the diagnosis of autoimmune thyroiditis, reveal the presence of nodules, or provide clues to the diagnosis of other thyroid conditions.[19]
Nuclear medicine
[edit]Nuclear imaging showing thyroid uptake can also be helpful in diagnosing thyroid function.
Misdiagnosis
[edit]Given the relatively nonspecific symptoms of initial hypothyroidism, Hashimoto's thyroiditis is often misdiagnosed as depression, cyclothymia, premenstrual syndrome, chronic fatigue syndrome, fibromyalgia, and less frequently, as erectile dysfunction or an anxiety disorder.
Treatment
[edit]There is no cure for Hashimoto's Thyroiditis. There is currently no known way to stop auto-immune lymphocytes infiltrating the thyroid or to regenerate thyroid tissue. However, the condition can be managed.
Managing hormone levels
[edit]
Hypothyroidism caused by Hashimoto's thyroiditis is treated with thyroid hormone replacement agents such as levothyroxine (T4), liothyronine (T3), or desiccated thyroid extract (T4+T3). A tablet taken once a day generally keeps the thyroid hormone levels normal. In most cases, the treatment needs to be taken for the rest of the person's life. If hypothyroidism is caused by Hashimoto's thyroiditis, the TSH levels may be recommended to be kept under 3.0 mIU/l.[62]
The standard of care is levothyroxine therapy, which is an oral medication identical in molecular structure to endogenous thyroxine.[17] Levothyroxine sodium has a sodium salt added to increase the gastrointestinal absorption of levothyroxine[63].
Levothyroxine dosing to normalise TSH is based on the amount of residual endogenous thyroid function and the patient’s weight, particularly lean body mass.[15] Usually the dose prescribed ranges from 1.6 mcg/kg to 1.8 mcg/kg, but can be adjusted based upon each patient.[10] For example, the dose may be lowered for elderly patients or patients with certain cardiac conditions, but should be increased in pregnant patients.[10] It should be administered on a consistent schedule.[17]
Side effects of thyroid replacement therapy are associated with iatrogenic hyperthyroidism.[17] Symptoms to watch out for include, but are not limited to, anxiety, tremor, weight loss, heat sensitivity, diarrhea, and shortness of breath. More worrisome symptoms include atrial fibrillation and bone density loss.[17]
Levothyroxine monotherapy vs Levothyroxine/Liothyronine combination therapy
[edit]Levothyroxine is preferred over Liothyronine due to its long half-life[24] leading to stable thyroid hormone levels, ease of monitoring, established body of evidence as an effective therapy in most people, and usefulness in pregnancy as it can cross the fetal blood brain barrier[15]. Some patients elect combination therapy with both levothyroxine and liothyronine, which is a synthetic T3, however studies of combination therapy are limited[17], and five meta-analyses/reviews "suggested no clear advantage of the combination therapy."[15] However, subgroup analysis found that patients who remain symptomatic while taking levothyroxine may benefit from therapy containing liothyronine.[15]
T4 to T3 conversion
[edit]Levothyroxine (T4) is the "storage form" of thyroid hormone; Liothyronine (T3) is the active form used by body tissues. In order for Levothyroxine therapy to succeed, the body must convert Levothyroxine into Liothyronine. Most conversion happens in the liver and gut, with some conversion occuring in the brain. Sufficient levels of the micronutrients Iron, Vitamin D, Vitamin A, Zinc and Selenium are necessary for adequate conversion. Conversion rates may decline with age. Patients with impaired conversion and no nutrient deficiencies may be recommended combination therapy of both levothyroxine and liothyronine.
Monitoring
[edit]Thyroid Stimulating Hormone (TSH) is the laboratory value of choice for monitoring response to treatment with levothyroxine.[61] When treatment is first initiated, TSH levels may be monitored as often as a frequency of every 6–8 weeks.[61] Each time the dose is adjusted, TSH levels may be measured at that frequency until the correct dose is determined.[61] Once titrated to a proper dose, TSH levels will be monitored yearly.[61]
Monitoring liothyronine treatment or combination treatment can be challenging. Liothyronine can suppress TSH to very low values. Liothyronine's short half-life can result in large fluctuations of free T3 over the course of 24 hours.
Other influences on thyroid hormone levels
[edit]Zinc may increase free T3 levels[64].
Improving wellbeing
[edit]Multiple studies have demonstrated persistent symptoms in euthyroid hashimotos patients[24][26]. Consequently, there has been some interest in therapies to improve wellbeing in euthyroid hashimotos patients, such as monitoring biomarkers that reflect the action of thyroid hormone on tissue levels.[15]
Three studies found Selenium may improve wellbeing and/or mood[65], one study found it did not improve wellbeing[66].
One study demonstrated surgical thyroid removal can substantially improve fatigue and wellbeing[67], see Surgery considerations.
Reducing antibodies
[edit]The benefits of reducing antithyroid antibodies in Hashimoto's are not established,[15] and at least one study has demonstrated a reduction in antibodies with no corresponding improvement in wellbeing.[66] While studies find that antibodies coincide with symptoms even in euthyroid patients[24][26], it has not been demonstrated that antibodies themselves cause symptoms. Antibodies are also not demonstrated to be reliable markers of immune attacks on the thyroid, as up to 10% of hashimotos patients do not have antibodies. Nevertheless, intervention studies frequently measure changes in antibody levels.
Selenium[68], Vitamin D[69], and Metformin[70] can reduce thyroid peroxidase antibodies. There is preliminary evidence that Levothyroxine[71][72], Aloe Vera Juice[73] and Black Cumin Seed[74] may reduce thyroid peroxidase antibodies. Metformin can reduce thyroglobulin antibodies.[70]
It is not established that a gluten-free diet can reduce antibodies when there is no comorbid coeliac disease.[75][64] Gluten free diets have been shown in several studies to reduce antibodies, and in other studies to have no effect, however there were significant confounding issues in these studies, including not ruling out comorbid coeliac disease.[75]
Surgical thyroid removal can substantially reduce anti-thyroid antibody levels[67], see Surgery considerations.
Surgery considerations
[edit]Surgery is not the initial treatment of choice for autoimmune disease, and uncomplicated Hashimoto's thyroiditis is not an indication for thyroidectomy.[17] Patients generally may discuss surgery with their doctor if they are experiencing significant pressure symptoms, or cosmetic concerns, or have nodules present on ultrasound.[17] One well-conducted study of patients with troublesome general symptoms and with anti-thyroperoxidase (anti-TPO) levels greater than 1000 IU/ml (normal <100 IU/ml) showed that total thyroidectomy caused the symptoms to resolve and median anti-thyroid peroxidase levels to reduce from 2232 to 152 IU/mL, but there were post-operative complications in 14%.[67]
Other
[edit]As of 2022, there are "no studies demonstrating the efficacy of low-dose naltrexone in autoimmune thyroid disorders and there is no evidence to support its use."[64] and "There are no studies showing that the elimination of dairy in patients with autoimmune thyroid disease who do not have lactose intolerance is of any clinical benefit."[64]
Prognosis
[edit]Overt, symptomatic thyroid dysfunction is the most common complication, with about 5% of people with subclinical hypothyroidism and chronic autoimmune thyroiditis progressing to thyroid failure every year. Transient periods of thyrotoxicosis (over-activity of the thyroid) sometimes occur, and rarely the illness may progress to full hyperthyroid Graves' disease with active orbitopathy (bulging, inflamed eyes).
Rare cases of fibrous autoimmune thyroiditis present with severe shortness of breath and difficulty swallowing, resembling aggressive thyroid tumors, but such symptoms always improve with surgery or corticosteroid therapy. Although primary thyroid B-cell lymphoma affects fewer than one in 1000 persons, it is more likely to affect those with long-standing autoimmune thyroiditis,[76] as there is a 67- to 80-fold increased risk of developing primary thyroid lymphoma in patients with Hashimoto's thyroiditis.[77]
Anti-thyroid antibodies
[edit]Thyroid peroxidase antibodies are observed to decline in patients treated with levothyroxine, with decreases varying between 10% and 90% after a follow-up of 6 to 24 months.[78] One study of patients treated with levothyroxine observed that 35 out of 38 patients (92%) had declines in thyroid peroxidase antibody levels over five years, lowering by 70% on average. 6 of the 38 patients (16%) had thyroid peroxidase antibody levels return to normal.[78]
Children
[edit]Many children diagnosed with hashimoto's disease will experience the same progressive course of the disease that adults do. However, of children who develop anti-thyroid antibodies and hypothyroidism, between 30-50% are later observed to have normal antibodies and thyroid hormone levels.[79][80]
One case of true remission has been observed in a 12 year old girl. Her thyroid was observed via ultrasound to progress from early inflammation to severe end-stage Hashimoto's thyroiditis with hypothyroidism, and then return to "almost normal with only minimal features of inflammation" and euthyroidism.[81]
Epidemiology
[edit]Hashimoto's Disease is estimated to affect 2% of the world's population.[17][29] About 1.0 to 1.5 in 1000 people have this disease at any time.[57]
Sex
[edit]Anyone may develop this disease, but it occurs between 8 and 15 times more often in women than in men[17]. Some research suggests a connection to the role of the placenta as an explanation for the sex difference.[82] The difference in prevalence amongst genders is due to the effects of sex hormones.[20]
High iodine consumption
[edit]Autoimmune thyroiditis has a higher prevalence in societies that have a higher intake of iodine in their diet, such as the United States and Japan, and among people who are genetically susceptible.[76] It is the most common cause of hypothyroidism in areas of sufficient iodine.[10] Also, the rate of lymphocytic infiltration increased in areas where the iodine intake was once low, but increased due to iodine supplementation.[28][83]
Iodine deficiency disorder (IDD) is combated using an increase in iodine in a person's diet. When a dramatic change occurs in a person's diet, they become more at-risk of developing hypothyroidism and other thyroid disorders. Combatting IDD with high salt intakes should be done carefully and cautiously as risk for Hashimoto's may increase.[83]
Geographic influence of dietary trends
[edit]Geography plays a large role in which regions have access to diets with low or high iodine. Iodine levels in both water and salt should be heavily monitored in order to protect at-risk populations from developing hypothyroidism.[84]
Geographic trends of hypothyroidism vary across the world as different places have different ways of defining disease and reporting cases. Populations that are spread out or defined poorly may skew data in unexpected ways.[29]
United States of America
[edit]Hashimoto's Thyroiditis may affect up to 5% of the United States' population.[85] Hashimoto's thyroiditis disorder is thought to be the most common cause of primary hypothyroidism in North America.[57]
Age
[edit]It has been shown that the prevalence of positive tests for thyroid antibodies increases with age, "with a frequency as high as 33 percent in women 70 years old or older."[28]
Hashimotos Thyroiditis can occur at any age, including children[76], but more commonly appears in middle age, particularly for men.[86] Incidence peaks in the fifth decade of life, but patients are usually diagnosed between age 30–50.[19][85] The highest prevalence from one study was found in the elderly members of the community.[87]
Race
[edit]About 5% of Caucasians will develop Hashimoto's at some point in their lives.[4] In the US, the African-American population experiences it less commonly but has greater associated mortality.[88] It is also less frequent in Asian populations.[89]
Autoimmune diseases
[edit]Those that already have an autoimmune disease are at greater risk of developing Hashimoto's as the diseases generally coexist with each other.[29] Common diseases seen coexisting with Hashimoto's include celiac disease, multiple sclerosis, type 1 diabetes, vitiligo, and rheumatoid arthritis.[citation needed]
Secular trends
[edit]The secular trends of hypothyroidism reveal how the disease has changed over the course of time given changes in technology and treatment options. Even though ultrasound technology and treatment options have improved, the incidence of hypothyroidism has increased according to data focused on the US and Europe. Between 1993 and 2001, per 1000 women, the disease was found varying between 3.9 and 4.89. Between 1994 and 2001, per 1000 men, the disease increased from 0.65 to 1.01.[87]
Changes in the definition of hypothyroidism and treatment options modify the incidence and prevalence of the disease overall. Treatment using levothyroxine is individualized, and therefore allows the disease to be more manageable with time but does not work as a cure for the disease.[29]
History
[edit]Also known as Hashimoto's disease, Hashimoto's thyroiditis is named after Japanese physician Hakaru Hashimoto (1881−1934) of the medical school at Kyushu University,[90] who first described the symptoms of persons with struma lymphomatosa, an intense infiltration of lymphocytes within the thyroid, in 1912 in the German journal called Archiv für Klinische Chirurgie.[3][91] This paper was made up of 30 pages and 5 illustrations all describing the histological changes in the thyroid tissue. Furthermore, all results in his first study were collected from four women. These results explained the pathological characteristics observed in these women especially the infiltration of lymphoid and plasma cells as well as the formation of lymphoid follicles with germinal centers, fibrosis, degenerated thyroid epithelial cells and leukocytes in the lumen.[3] He described these traits to be histologically similar to those of Mikulic's disease. As mentioned above, once he discovered these traits in this new disease, he named the disease struma lymphomatosa. This disease emphasized the lymphoid cell infiltration and formation of the lymphoid follicles with germinal centers, neither of which had ever been previously reported.[3]
Despite Dr. Hashimoto's discovery and publication, the disease was not recognized as distinct from Reidel's thyroiditis, which was a common disease at that time in Europe. Although many other articles were reported and published by other researchers, Hashimoto's struma lymphomatosa was only recognized as an early phase of Reidel's thyroiditis in the early 1900s. It was not until 1931 that the disease was recognized as a disease in its own right, when researchers Allen Graham et al. from Cleveland reported its symptoms and presentation in the same detailed manner as Hakaru.[3]
In 1956, Drs. Rose and Witebsky were able to demonstrate how immunization of certain rodents with extracts of other rodents' thyroid resembled the disease Hakaru and other researchers were trying to describe.[3] These doctors were also able to describe anti-thyroglobulin antibodies in blood serum samples from these same animals.[citation needed]
Later on in the same year, researchers from the Middlesex Hospital in London were able to perform human experiments on patients who presented with similar symptoms. They purified anti-thyroglobulin antibody from their serum and were able to conclude that these sick patients had an immunological reaction to human thyroglobulin.[3] From this data, it was proposed that Hashimoto's struma could be an autoimmune disease of the thyroid gland.
In 1957, it was recognized as an autoimmune disorder and was the first organ-specific autoimmune disorder identified.[12]
Following this recognition, the same researchers from Middlesex Hospital published an article in 1962 in The Lancet that included a portrait of Hakaru Hashimoto.[3] The disease became more well known from that moment, and Hashimoto's disease started to appear more frequently in textbooks.[92]
Pregnancy
[edit]Conception
[edit]It is recommended that overt hypothyroidism be treated with levothyoxine to reach euthyroidism before conception, in order "to prevent adverse effects on pregnancy and child developmental outcomes".[15] The embryo transplantation rate is improved when hypothyroidism is treated.[93]
Pregnancy
[edit]The Endocrine Society recommends screening in pregnant women who are considered high-risk for thyroid autoimmune disease.[94] Universal screening for thyroid diseases during pregnancy is controversial, however, one study "supports the potential benefit of universal screening".[95]
Pregnant women may have anti-thyroid antibodies (5%–14% of pregnancies[15]), poor thyroid function resulting in hypothyroidism, or both. Each is associated with risks.[15]
Anti-thyroid antibodies in pregnancy
[edit]The presence of Thyroid Peroxidase antibodies at the outset of pregnancy are associated with a greater risk to the mother of hypothyroidism and thyroid impairment in the first year after delivery.[96]
The presence of antibodies is also associated with "a 2 to 4-fold increase in the risk of recurrent miscarriages, and 2 to 3- fold increased risk of preterm birth.", however the reason why is unclear. Thyroid Peroxidase antibodies are speculated to indicate other autoimmune processes against the placental-fetal unit.[15]
Levothyroxine treatment in euthyroid women with thyroid autoimmunity does not significantly impact the relative risk of miscarriage and preterm delivery, or outcomes with live birth. "Therefore, no strong recommendations regarding the therapy in such scenarios could be made, but consideration on a case-by-case basis might be implemented."[15]
Hypothyroidism in pregnancy.
[edit]Women who have low thyroid function that has not been stabilized are at greater risk of complications for both parent and child. Risks to the mother include gestational hypertension including preeclampsia and eclampsia, gestational diabetes, placental abruption, and postpartum hemorrhage[15]. Risks to the infant include miscarriage, preterm delivery, low birth weight, neonatal respiratory distress, hydrocephalus, hypospadias, fetal death, infant intensive care unit admission, and neurodevelopmental delays (lower child IQ, language delay or global developmental delay).[95][93][15]
Successful pregnancy outcomes are improved when hypothyroidism is treated.[93] Levothyroxine treatment may be considered at lower TSH levels in pregnancy than in standard treatment.[15] Liothyronine does not cross the fetal blood-brain barrier, so liothyronine (T3) only or liothyronine + levothyroxine (T3 + T4) therapy is not indicated in pregnancy.[15]
Close cooperation between the endocrinologist and obstetrician benefits the woman and the infant.[95][97][98]
Immune changes during pregnancy
[edit]Hormonal changes and trophoblast expression of key immunomodulatory molecules lead to immunosuppression and fetal tolerance. Main players in regulation of the immune response are Tregs. Both cell-mediated and humoral immune responses are attenuated, resulting in immune tolerance and suppression of autoimmunity. It has been reported that during pregnancy, levels of thyroid peroxidase and thyroglobulin antibodies decrease.[99]
Postpartum
[edit]Postpartum thyroiditis can occur up to 1 year after delivery in healthy women and should be differentiated from Hashimoto's thyroiditis as it is treated differently.[100]
Thyroid peroxidase antibodies testing is recommended for women who have ever been pregnant regardless of pregnancy outcome. "[P]revious pregnancy plays a major role in development of autoimmune overt hypothyroidism in premenopausal women, and the number of previous pregnancies should be taken into account when evaluating the risk of hypothyroidism in a young women [sic]."[51]
After giving birth, Tregs rapidly decrease and immune responses are re-established. It may lead to the occurrence or aggravation of the autoimmune thyroid disease.[99] In up to 50% of females with thyroid peroxidase antibodies in the early pregnancy, thyroid autoimmunity in the postpartum period exacerbates in the form of postpartum thyroiditis.[101] Higher secretion of IFN-γ and IL-4, and lower plasma cortisol concentration during pregnancy has been reported in females with postpartum thyroiditis than in healthy females. It indicates that weaker immunosuppression during pregnancy could contribute to the postpartum thyroid dysfunction.[102]
Fetal microchimerism
[edit]Several years after the delivery, the chimeric male cells can be detected in the maternal peripheral blood, thyroid, lung, skin, or lymph nodes. The fetal immune cells in the maternal thyroid gland may become activated and act as a trigger that may initiate or exaggerate the autoimmune thyroid disease. In Hashimoto's disease patients, fetal microchimeric cells were detected in thyroid in significantly higher numbers than in healthy females.[103]
Other organisms
[edit]Hashimoto's disease is also known in chickens (Gallus domesticus),[104][105] rats (Rattus rattus),[105] mice (Mus musculus),[105] dogs (Canis familiaris),[105] and marmosets (Callitrichidae).[105]
Pseudoscience
[edit]Pseudoscientific claims and "rogue practitioners" pose increasing risks to patients.[106]
"We have seen practitioners who proclaim themselves to be experts in hormonal therapy without any formal training and who often promote hormonal treatments without adequate endocrine evaluations. We have seen practitioners who make astonishing promises regarding the benefits of herbal, supplemental, and other unproven therapies that they themselves sell in their offices and/or online. And we have seen what we know to be frankly harmful and even dangerous products that contain animal whole organ (most commonly thyroid and/or adrenal) extracts or hormonal injections that produce highly elevated levels of sex hormones (especially testosterone) without any concern for short-term patient safety or longterm outcomes. And we have heard anecdotal stories from patients who visited these practitioners and had no beneficial results or frankly concerning on-treatment results at a surprisingly high financial cost, even though they had been promised symptom improvement, safety, and full insurance coverage."[106]
See also
[edit]References
[edit]- ^ a b c d e f g h i j k l m n o p "Hashimoto's Disease". NIDDK. May 2014. Archived from the original on 22 August 2016. Retrieved 9 August 2016.
- ^ a b Noureldine SI, Tufano RP (January 2015). "Association of Hashimoto's thyroiditis and thyroid cancer". Current Opinion in Oncology. 27 (1): 21–25. doi:10.1097/cco.0000000000000150. PMID 25390557. S2CID 32109200.
- ^ a b c d e f g h i Hiromatsu Y, Satoh H, Amino N (January 2013). "Hashimoto's thyroiditis: history and future outlook". Hormones. 12 (1): 12–18. doi:10.1007/BF03401282. PMID 23624127. S2CID 38996783.
- ^ a b c d e Pyzik A, Grywalska E, Matyjaszek-Matuszek B, Roliński J (2015). "Immune disorders in Hashimoto's thyroiditis: what do we know so far?". Journal of Immunology Research. 2015: 979167. doi:10.1155/2015/979167. PMC 4426893. PMID 26000316.
- ^ a b c d e Akamizu T, Amino N, Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland J, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, Wilson DP (2000). "Hashimoto's Thyroiditis". In Akamizu T, Amino N (eds.). Endotext. MDText. PMID 25905412. Archived from the original on 24 September 2020. Retrieved 31 January 2021.
- ^ "Autoimmune thyroiditis". Autoimmune Registry Inc. Archived from the original on 25 February 2020. Retrieved 15 June 2022.
- ^ a b "Hashimoto's Disease - NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Retrieved 4 December 2024.
- ^ "Hashimoto Thyroiditis – Endocrine and Metabolic Disorders". Merck Manuals Professional Edition. July 2016. Archived from the original on 31 December 2017. Retrieved 30 December 2017.
- ^ "Hashimoto Thyroiditis – Hormonal and Metabolic Disorders". Merck Manuals Consumer Version. Archived from the original on 30 December 2017. Retrieved 30 December 2017.
- ^ a b c d e f g h i j Mincer DL, Jialal I (2022), "Hashimoto Thyroiditis", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 29083758, archived from the original on 4 October 2023, retrieved 23 January 2023
- ^ Shoenfeld Y, Cervera R, Gershwin ME, eds. (2010). Diagnostic Criteria in Autoimmune Diseases. Springer Science & Business Media. p. 216. ISBN 978-1-60327-285-8.
- ^ a b Moore EA, Wilkinson S (2009). The Promise of Low Dose Naltrexone Therapy: Potential Benefits in Cancer, Autoimmune, Neurological and Infectious Disorders. McFarland. p. 30. ISBN 978-0-7864-5258-3.
- ^ a b c d e f Singh S, Clutter WE (2020). The Washington Manual®, The Endocrinology - Subspecialty Consult (4th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 70–76. ISBN 978-1-9751-1333-9.
- ^ Page 56 in: Staecker H, Van De Water TR (2006). Otolaryngology: basic science and clinical review. Stuttgart: Thieme. ISBN 978-0-86577-901-3.
- ^ a b c d e f g h i j k l m n o p q r Klubo-Gwiezdzinska J, Wartofsky L (30 March 2022). "Hashimoto thyroiditis: an evidence-based guide to etiology, diagnosis and treatment". Polish Archives of Internal Medicine. 132 (3): 16222. doi:10.20452/pamw.16222. ISSN 0032-3772. PMC 9478900. PMID 35243857.
- ^ "Pathogenesis of Hashimoto's thyroiditis (chronic autoimmune thyroiditis)". UpToDate. Archived from the original on 5 December 2020. Retrieved 19 July 2019.
- ^ a b c d e f g h i j k l m n o p q r s t Ramos-Levi AM, Marazuela M (2023). DeGroot's Endocrinology, Basic Science and Clinical Practice (8th ed.). Philadelphia, PA: Elsevier. pp. 1214–1233. ISBN 978-0-323694124.
- ^ Fariduddin MM, Haq N, Bansal N (2024), "Hypothyroid Myopathy", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30137798, retrieved 30 November 2024
- ^ a b c d e f g h "Hashimoto's Disease | NIDDK". National Institute of Diabetes and Digestive and Kidney Diseases. Archived from the original on 8 December 2021. Retrieved 23 January 2023.
- ^ a b c d e Weetman AP (2021). Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text (11th ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 531–541. ISBN 978-1-975112-96-7.
- ^ "Hashimoto's disease – Symptoms and causes". Mayo Clinic. Archived from the original on 29 October 2018. Retrieved 5 October 2018.
- ^ Fariduddin MM, Haq N, Bansal N (2024), "Hypothyroid Myopathy", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30137798, retrieved 30 November 2024
- ^ Mincer DL, Jialal I (2019). "Hashimoto Thyroiditis". NCBI StatPearls. PMID 29083758. Archived from the original on 23 November 2019. Retrieved 19 July 2019.
- ^ a b c d Groenewegen KL, Mooij CF, van Trotsenburg AP (2021). "Persisting symptoms in patients with Hashimoto's disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review". Journal of Translational Autoimmunity. 4: 100101. doi:10.1016/j.jtauto.2021.100101. ISSN 2589-9090. PMC 8122172. PMID 34027377.
- ^ Jordan B, Uer O, Buchholz T, Spens A, Zierz S (July 2021). "Physical fatigability and muscle pain in patients with Hashimoto thyroiditis". Journal of Neurology. 268 (7): 2441–2449. doi:10.1007/s00415-020-10394-5. ISSN 1432-1459. PMC 8217009. PMID 33507372.
- ^ a b c Li J, Huang Q, Sun S, Zhou K, Wang X, Pan K, Zhang Y, Wang Y, Han Q, Si C, Li S, Fan S, Li D (13 November 2024). "Thyroid antibodies in Hashimoto's thyroiditis patients are positively associated with inflammation and multiple symptoms". Scientific Reports. 14 (1): 27902. Bibcode:2024NatSR..1427902L. doi:10.1038/s41598-024-78938-7. ISSN 2045-2322. PMC 11561229. PMID 39537841.
- ^ a b Casto C, Pepe G, Li Pomi A, Corica D, Aversa T, Wasniewska M (2021). "Hashimoto's Thyroiditis and Graves' Disease in Genetic Syndromes in Pediatric Age". Genes. 12 (2): 222. doi:10.3390/genes12020222. ISSN 2073-4425. PMC 7913917. PMID 33557156.
- ^ a b c d Dayan Dayan, Colin M, Dayan, Colin M., Gilbert H. Daniels (1996). "Chronic Autoimmune Thyroiditis". The New England Journal of Medicine. 335 (2): 99–107. doi:10.1056/nejm199607113350206. PMID 8649497.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ a b c d e f Chistiakov DA (March 2005). "Immunogenetics of Hashimoto's thyroiditis". Journal of Autoimmune Diseases. 2 (1): 1. doi:10.1186/1740-2557-2-1. PMC 555850. PMID 15762980.
- ^ a b Jacobson EM, Huber A, Tomer Y (2008). "The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology". Journal of Autoimmunity. 30 (1–2): 58–62. doi:10.1016/j.jaut.2007.11.010. PMC 2244911. PMID 18178059.
- ^ Tandon N, Zhang L, Weetman AP (May 1991). "HLA associations with Hashimoto's thyroiditis". Clinical Endocrinology. 34 (5): 383–386. doi:10.1111/j.1365-2265.1991.tb00309.x. PMID 1676351. S2CID 28987581.
- ^ Bogner U, Badenhoop K, Peters H, Schmieg D, Mayr WR, Usadel KH, Schleusener H (January 1992). "HLA-DR/DQ gene variation in nongoitrous autoimmune thyroiditis at the serological and molecular level". Autoimmunity. 14 (2): 155–158. doi:10.3109/08916939209083135. PMID 1363895.
- ^ Zaletel K, Gaberšček S (December 2011). "Hashimoto's Thyroiditis: From Genes to the Disease". Current Genomics. 12 (8): 576–588. doi:10.2174/138920211798120763. PMC 3271310. PMID 22654557.
- ^ Tomer Y, Greenberg DA, Barbesino G, Concepcion E, Davies TF (April 2001). "CTLA-4 and not CD28 is a susceptibility gene for thyroid autoantibody production". The Journal of Clinical Endocrinology and Metabolism. 86 (4): 1687–1693. doi:10.1210/jcem.86.4.7372. PMID 11297604.
- ^ Ban Y, Davies TF, Greenberg DA, Kissin A, Marder B, Murphy B, et al. (December 2003). "Analysis of the CTLA-4, CD28, and inducible costimulator (ICOS) genes in autoimmune thyroid disease". Genes and Immunity. 4 (8): 586–593. doi:10.1038/sj.gene.6364018. PMID 14647199. S2CID 6920190.
- ^ Burn GL, Svensson L, Sanchez-Blanco C, Saini M, Cope AP (December 2011). "Why is PTPN22 a good candidate susceptibility gene for autoimmune disease?". FEBS Letters. 585 (23): 3689–3698. Bibcode:2011FEBSL.585.3689B. doi:10.1016/j.febslet.2011.04.032. PMID 21515266. S2CID 21572847.
- ^ Ito C, Watanabe M, Okuda N, Watanabe C, Iwatani Y (August 2006). "Association between the severity of Hashimoto's disease and the functional +874A/T polymorphism in the interferon-gamma gene". Endocrine Journal. 53 (4): 473–478. doi:10.1507/endocrj.k06-015. PMID 16820703.
- ^ Nanba T, Watanabe M, Akamizu T, Iwatani Y (March 2008). "The -590CC genotype in the IL4 gene as a strong predictive factor for the development of hypothyroidism in Hashimoto disease". Clinical Chemistry. 54 (3): 621–623. doi:10.1373/clinchem.2007.099739. PMID 18310157.
- ^ Yamada H, Watanabe M, Nanba T, Akamizu T, Iwatani Y (March 2008). "The +869T/C polymorphism in the transforming growth factor-beta1 gene is associated with the severity and intractability of autoimmune thyroid disease". Clinical and Experimental Immunology. 151 (3): 379–382. doi:10.1111/j.1365-2249.2007.03575.x. PMC 2276968. PMID 18190611.
- ^ Inoue N, Watanabe M, Morita M, Tomizawa R, Akamizu T, Tatsumi K, et al. (December 2010). "Association of functional polymorphisms related to the transcriptional level of FOXP3 with prognosis of autoimmune thyroid diseases". Clinical and Experimental Immunology. 162 (3): 402–406. doi:10.1111/j.1365-2249.2010.04229.x. PMC 3026543. PMID 20942809.
- ^ Inoue N, Watanabe M, Nanba T, Wada M, Akamizu T, Iwatani Y (May 2009). "Involvement of functional polymorphisms in the TNFA gene in the pathogenesis of autoimmune thyroid diseases and production of anti-thyrotropin receptor antibody". Clinical and Experimental Immunology. 156 (2): 199–204. doi:10.1111/j.1365-2249.2009.03884.x. PMC 2759465. PMID 19250279.
- ^ Hansen PS, Brix TH, Iachine I, Kyvik KO, Hegedüs L (January 2006). "The relative importance of genetic and environmental effects for the early stages of thyroid autoimmunity: a study of healthy Danish twins". European Journal of Endocrinology. 154 (1): 29–38. doi:10.1530/eje.1.02060. PMID 16381988. S2CID 25372591.
- ^ McCombe PA, Greer JM, Mackay IR (December 2009). "Sexual dimorphism in autoimmune disease". Current Molecular Medicine. 9 (9): 1058–1079. doi:10.2174/156652409789839116. PMID 19747114.
- ^ Invernizzi P, Miozzo M, Selmi C, Persani L, Battezzati PM, Zuin M, et al. (July 2005). "X chromosome monosomy: a common mechanism for autoimmune diseases". Journal of Immunology. 175 (1): 575–578. doi:10.4049/jimmunol.175.1.575. PMID 15972694. S2CID 40557667.
- ^ a b c d e f g Surks MI, Sievert R (December 1995). Wood AJ (ed.). "Drugs and thyroid function". The New England Journal of Medicine. 333 (25): 1688–1694. doi:10.1056/NEJM199512213332507. PMID 7477223.
- ^ Rose NR, Bonita R, Burek CL (February 2002). "Iodine: an environmental trigger of thyroiditis". Autoimmunity Reviews. 1 (1–2): 97–103. CiteSeerX 10.1.1.326.5700. doi:10.1016/s1568-9972(01)00016-7. PMID 12849065.
- ^ Burek CL, Talor MV (November 2009). "Environmental triggers of autoimmune thyroiditis". Journal of Autoimmunity. 33 (3–4): 183–189. doi:10.1016/j.jaut.2009.09.001. PMC 2790188. PMID 19818584.
- ^ Fountoulakis S, Philippou G, Tsatsoulis A (January 2007). "The role of iodine in the evolution of thyroid disease in Greece: from endemic goiter to thyroid autoimmunity". Hormones. 6 (1): 25–35. PMID 17324915.
- ^ Yu X, Li L, Li Q, Zang X, Liu Z (November 2011). "TRAIL and DR5 promote thyroid follicular cell apoptosis in iodine excess-induced experimental autoimmune thyroiditis in NOD mice". Biological Trace Element Research. 143 (2): 1064–1076. Bibcode:2011BTER..143.1064Y. doi:10.1007/s12011-010-8941-5. PMID 21225479. S2CID 10926594.
- ^ Pedersen IB, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Laurberg P (January 2003). "Thyroid peroxidase and thyroglobulin autoantibodies in a large survey of populations with mild and moderate iodine deficiency". Clinical Endocrinology. 58 (1): 36–42. doi:10.1046/j.1365-2265.2003.01633.x. PMID 12519410. S2CID 23758580.
- ^ a b Carlé A, Pedersen IB, Knudsen N, Perrild H, Ovesen L, Rasmussen LB, Laurberg P (June 2014). "Development of autoimmune overt hypothyroidism is highly associated with live births and induced abortions but only in premenopausal women". The Journal of Clinical Endocrinology and Metabolism. 99 (6): 2241–2249. doi:10.1210/jc.2013-4474. PMID 24694338.
- ^ Radetti G (2014). "Clinical Aspects of Hashimoto's Thyroiditis". Paediatric Thyroidology. Endocrine Development. Vol. 26. pp. 158–170. doi:10.1159/000363162. ISBN 978-3-318-02720-4. PMID 25231451.
- ^ Saranac L, Zivanovic S, Bjelakovic B, Stamenkovic H, Novak M, Kamenov B (2011). "Why is the thyroid so prone to autoimmune disease?". Hormone Research in Paediatrics. 75 (3): 157–165. doi:10.1159/000324442. PMID 21346360.
- ^ a b Mikulska AA, Karaźniewicz-Łada M, Filipowicz D, Ruchała M, Główka FK (January 2022). "Metabolic Characteristics of Hashimoto's Thyroiditis Patients and the Role of Microelements and Diet in the Disease Management—An Overview". International Journal of Molecular Sciences. 23 (12): 6580. doi:10.3390/ijms23126580. ISSN 1422-0067. PMC 9223845. PMID 35743024.
- ^ a b Berghi N (2017). "Immunological Mechanisms Implicated in the Pathogenesis of Chronic Urticaria and Hashimoto Thyroiditis". Iranian Journal of Allergy, Asthma and Immunology. 16 (4): 358–366. PMID 28865416. Archived from the original on 19 April 2021. Retrieved 3 December 2020.
- ^ "Thyroid Antibodies". Archived from the original on 19 July 2011. Retrieved 4 April 2012.
- ^ a b c d e Maitra A (2014). "The Endocrine System". In Kumar V, Abbas AK, Aster JC (eds.). Robbins and Cotran Pathologic Basis of Disease. Elsevier Health Sciences. pp. 1073–1140. ISBN 978-0-323-29635-9.
- ^ Dayan CM, Daniels GH (July 1996). "Chronic autoimmune thyroiditis". The New England Journal of Medicine. 335 (2): 99–107. doi:10.1056/NEJM199607113350206. PMID 8649497.
- ^ Grani G, Carbotta G, Nesca A, D'Alessandri M, Vitale M, Del Sordo M, Fumarola A (June 2015). "A comprehensive score to diagnose Hashimoto's thyroiditis: a proposal". Endocrine. 49 (2): 361–365. doi:10.1007/s12020-014-0441-5. PMID 25280964. S2CID 23026213.
- ^ Hashimoto Thyroiditis~workup at eMedicine
- ^ a b c d e "Hashimoto's Thyroiditis". American Thyroid Association. Archived from the original on 23 September 2023. Retrieved 23 January 2023.
- ^ "Does Your Doctor Know About the New TSH Lab Standards?". Archived from the original on 4 December 2010.
- ^ Wiersinga WM (2016). Endocrinology: Adult and Pediatric. Vol. 2 (7th ed.). pp. 1540–1556.
- ^ a b c d Larsen D, Singh S, Brito M (11 August 2022). "Thyroid, Diet, and Alternative Approaches". The Journal of Clinical Endocrinology & Metabolism. 107 (11): 2973–2981.
- ^ Toulis KA, Anastasilakis AD, Tzellos TG, Goulis DG, Kouvelas D (2010). "Selenium supplementation in the treatment of Hashimoto's thyroiditis: a systematic review and a meta-analysis". Thyroid: Official Journal of the American Thyroid Association. 20 (10): 1163–1173. doi:10.1089/thy.2009.0351. ISSN 1557-9077. PMID 20883174.
- ^ a b Mantovani G, Isidori AM, Moretti C, Di Dato C, Greco E, Ciolli P, Bonomi M, Petrone L, Fumarola A, Campagna G, Vannucchi G, Di Sante S, Pozza C, Faggiano A, Lenzi A (1 December 2019). "Selenium supplementation in the management of thyroid autoimmunity during pregnancy: results of the "SERENA study", a randomized, double-blind, placebo-controlled trial". Endocrine. 66 (3): 542–550. doi:10.1007/s12020-019-01958-1. ISSN 1559-0100. PMID 31129812.
- ^ a b c Guldvog I et al. Annals of Internal Medicine 2019; 21: 161-167.
- ^ Huwiler VV, Maissen-Abgottspon S, Stanga Z, Mühlebach S, Trepp R, Bally L, Bano A (March 2024). "Selenium Supplementation in Patients with Hashimoto Thyroiditis: A Systematic Review and Meta-Analysis of Randomized Clinical Trials". Thyroid: Official Journal of the American Thyroid Association. 34 (3): 295–313. doi:10.1089/thy.2023.0556. ISSN 1557-9077. PMC 10951571. PMID 38243784.
- ^ Jiang H, Chen X, Qian X, Shao S (2022). "Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto's thyroiditis—A meta-analysis of randomized controlled trials". Journal of Clinical Pharmacy and Therapeutics. 47 (6): 767–775. doi:10.1111/jcpt.13605. ISSN 1365-2710. PMC 9302126. PMID 34981556.
- ^ a b Jia X, Zhai T, Zhang Ja (17 August 2020). "Metformin reduces autoimmune antibody levels in patients with Hashimoto's thyroiditis: A systematic review and meta-analysis". Autoimmunity. 53 (6): 353–361. doi:10.1080/08916934.2020.1789969. ISSN 0891-6934. PMID 32741222.
- ^ Aksoy DY, Kerimoglu U, Okur H, Canpinar H, Karaagaoglu E, Yetgin S, Kansu E, Gedik O (2005). "Effects of Prophylactic Thyroid Hormone Replacement in Euthyroid Hashimoto's Thyroiditis". Endocrine Journal. 52 (3): 337–343. doi:10.1507/endocrj.52.337.
- ^ Padberg S, Heller K, Usadel K, Schumm-Draeger PM (March 2001). "One-Year Prophylactic Treatment of Euthyroid Hashimoto's Thyroiditis Patients with Levothyroxine: Is There a Benefit?". Thyroid®. 11 (3): 249–255. doi:10.1089/105072501750159651. ISSN 1050-7256.
- ^ Metro D, Cernaro V, Papa M, Benvenga S (1 March 2018). "Marked improvement of thyroid function and autoimmunity by Aloe barbadensis miller juice in patients with subclinical hypothyroidism". Journal of Clinical & Translational Endocrinology. 11: 18–25. doi:10.1016/j.jcte.2018.01.003. ISSN 2214-6237. PMC 5842288. PMID 29527506.
- ^ Osowiecka K, Myszkowska-Ryciak J (January 2023). "The Influence of Nutritional Intervention in the Treatment of Hashimoto's Thyroiditis—A Systematic Review". Nutrients. 15 (4): 1041. doi:10.3390/nu15041041. ISSN 2072-6643. PMC 9962371. PMID 36839399.
- ^ a b Szczuko M, Syrenicz A, Szymkowiak K, Przybylska A, Szczuko U, Pobłocki J, Kulpa D (2022). "Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto's Disease". Nutrients. 14 (9): 1727. doi:10.3390/nu14091727. ISSN 2072-6643. PMC 9101474. PMID 35565695.
- ^ a b c Monaco F (2012). Thyroid Diseases. Taylor and Francis. p. 78. ISBN 978-1-4398-6839-3.
- ^ Noureldine SI, Tufano RP (January 2015). "Association of Hashimoto's thyroiditis and thyroid cancer". Current Opinion in Oncology. 27 (1): 21–25. doi:10.1097/CCO.0000000000000150. PMID 25390557. S2CID 32109200.
- ^ a b Schmidt M, Voell M, Rahlff I, Dietlein M, Kobe C, Faust M, Schicha H (2008). "Long-term follow-up of antithyroid peroxidase antibodies in patients with chronic autoimmune thyroiditis (Hashimoto's thyroiditis) treated with levothyroxine". Thyroid: Official Journal of the American Thyroid Association. 18 (7): 755–760. doi:10.1089/thy.2008.0008. ISSN 1050-7256. PMID 18631004.
- ^ Demirbilek H, Kandemir N, Gonc EN, Ozon A, Alikasifoglu A (2009). "Assessment of thyroid function during the long course of Hashimoto's thyroiditis in children and adolescents". Clinical Endocrinology. 71 (3): 451–454. doi:10.1111/j.1365-2265.2008.03501.x. ISSN 1365-2265. PMID 19094075.
- ^ Marković S, Kostić G, Igrutinović Z, Vuletić B (2008). "Hashimoto's thyroiditis in children and adolescents". Srpski Arhiv Za Celokupno Lekarstvo. 136 (5–6): 262–266. doi:10.2298/SARH0806262M. PMID 18792623.
- ^ Nanan R, Wall JR (2010). "Remission of Hashimoto's thyroiditis in a twelve-year-old girl with thyroid changes documented by ultrasonography". Thyroid: Official Journal of the American Thyroid Association. 20 (10): 1187–1190. doi:10.1089/thy.2010.0102. ISSN 1557-9077. PMID 20883175.
- ^ Natri H, Garcia AR, Buetow KH, Trumble BC, Wilson MA (July 2019). "The Pregnancy Pickle: Evolved Immune Compensation Due to Pregnancy Underlies Sex Differences in Human Diseases". Trends in Genetics. 35 (7): 478–488. doi:10.1016/j.tig.2019.04.008. PMC 6611699. PMID 31200807.
- ^ a b Khattak RM, Ittermann T, Nauck M, Below H, Völzke H (2016). "Monitoring the prevalence of thyroid disorders in the adult population of Northeast Germany". Population Health Metrics. 14: 39. doi:10.1186/s12963-016-0111-3. PMC 5101821. PMID 27833458.
- ^ Katagiri R, Yuan X, Kobayashi S, Sasaki S (10 March 2017). "Effect of excess iodine intake on thyroid diseases in different populations: A systematic review and meta-analyses including observational studies". PLOS ONE. 12 (3): e0173722. Bibcode:2017PLoSO..1273722K. doi:10.1371/journal.pone.0173722. PMC 5345857. PMID 28282437.
- ^ a b Biddinger PW (2020). Diagnostic Pathology and Molecular Genetics of the Thyroid: A Comprehensive Guide for Practicing Thyroid Pathology (3rd ed.). Philadelphia, PA: Lippincott Williams & Wilkins. pp. 59–72. ISBN 978-1-4963-9653-2.
- ^ "Hashimoto's disease fact sheet". Office on Women's Health, U.S. Department of Health and Human Services, womenshealth.gov (or girlshealth.gov). 16 July 2012. Archived from the original on 2 December 2014. Retrieved 23 November 2014.
- ^ a b Vanderpump MP (1 September 2011). "The epidemiology of thyroid disease". British Medical Bulletin. 99 (1): 39–51. doi:10.1093/bmb/ldr030. PMID 21893493.
- ^ Boyles S (23 May 2013). "Hypothyroidism Hikes Death Risk in Blacks". MedPage Today.
- ^ McLeod DS, Caturegli P, Cooper DS, Matos PG, Hutfless S (April 2014). "Variation in rates of autoimmune thyroid disease by race/ethnicity in US military personnel". JAMA. 311 (15): 1563–1565. doi:10.1001/jama.2013.285606. PMID 24737370.
- Lay summary in: McLeod DS (15 April 2014). "Thyroid Disease Risk Varies Among Blacks, Asians, and Whites". JAMA Network.
- ^ Hakaru Hashimoto at Who Named It?
- ^ Hashimoto H (1912). "Zur Kenntnis der lymphomatösen Veränderung der Schilddrüse (Struma lymphomatosa)" [Knowledge of lymphomatous changes in the thyroid gland (goiter lymphomatosa)]. Archiv für Klinische Chirurgie (in German). 97: 219–248. NAID 10005555208.
- ^ "Google Books Ngram Viewer". books.google.com. Retrieved 1 December 2024.
- ^ a b c Gaberšček S, Zaletel K (September 2011). "Thyroid physiology and autoimmunity in pregnancy and after delivery". Expert Review of Clinical Immunology. 7 (5): 697–706, quiz 707. doi:10.1586/eci.11.42. PMID 21895480.
- ^ "Endocrine Experts Support Screening for Thyroid Dysfunction in Pregnant Women". Endocrine Society. 26 March 2015. Archived from the original on 8 October 2015. Retrieved 4 October 2015.
- ^ a b c Lepoutre T, Debiève F, Gruson D, Daumerie C (1 January 2012). "Reduction of miscarriages through universal screening and treatment of thyroid autoimmune diseases". Gynecologic and Obstetric Investigation. 74 (4): 265–273. doi:10.1159/000343759. PMID 23147711. S2CID 1646888.
- ^ Caturegli P, De Remigis A, Rose NR (1 April 2014). "Hashimoto thyroiditis: Clinical and diagnostic criteria". Autoimmunity Reviews. Diagnostic criteria in Autoimmune diseases. 13 (4): 391–397. doi:10.1016/j.autrev.2014.01.007. ISSN 1568-9972. PMID 24434360.
- ^ Budenhofer BK, Ditsch N, Jeschke U, Gärtner R, Toth B (January 2013). "Thyroid (dys-)function in normal and disturbed pregnancy". Archives of Gynecology and Obstetrics. 287 (1): 1–7. doi:10.1007/s00404-012-2592-z. PMID 23104052. S2CID 24969196. Archived from the original on 23 January 2022. Retrieved 16 January 2022.
- ^ Balucan FS, Morshed SA, Davies TF (2013). "Thyroid autoantibodies in pregnancy: their role, regulation and clinical relevance". Journal of Thyroid Research. 2013: 182472. doi:10.1155/2013/182472. PMC 3652173. PMID 23691429.
- ^ a b Weetman AP (June 2010). "Immunity, thyroid function and pregnancy: molecular mechanisms". Nature Reviews. Endocrinology. 6 (6): 311–318. doi:10.1038/nrendo.2010.46. PMID 20421883. S2CID 9900120.
- ^ Lee SY, Pearce EN (2022). "Assessment and treatment of thyroid disorders in pregnancy and the postpartum period". Nature Reviews Endocrinology. 18 (3): 158–171. doi:10.1038/s41574-021-00604-z. ISSN 1759-5037. PMC 9020832. PMID 34983968.
- ^ Lazarus JH (March 2011). "The continuing saga of postpartum thyroiditis". The Journal of Clinical Endocrinology and Metabolism. 96 (3): 614–616. doi:10.1210/jc.2011-0091. PMID 21378224.
- ^ Kokandi AA, Parkes AB, Premawardhana LD, John R, Lazarus JH (March 2003). "Association of postpartum thyroid dysfunction with antepartum hormonal and immunological changes". The Journal of Clinical Endocrinology and Metabolism. 88 (3): 1126–1132. doi:10.1210/jc.2002-021219. PMID 12629095.
- ^ Koopmans M, Kremer Hovinga IC, Baelde HJ, Harvey MS, de Heer E, Bruijn JA, Bajema IM (June 2008). "Chimerism occurs in thyroid, lung, skin and lymph nodes of women with sons". Journal of Reproductive Immunology. 78 (1): 68–75. doi:10.1016/j.jri.2008.01.002. PMID 18329105.
- ^ Wick G, Möst J, Schauenstein K, Krömer G, Dietrich H, Ziemiecki A, Fässler R, Schwarz S, Eu N, Hálaa K (1985). "Spontaneous autoimmune thyroiditis - a bird's eye view". Immunology Today. 12 (6). Elsevier Science Publishers B.V.: 359–64. doi:10.1016/0167-5699(85)90095-7. PMID 25291225. S2CID 8191727. Archived from the original on 29 December 2022. Retrieved 29 December 2022.
- ^ a b c d e McLachlan S, Alpi K, Rapoport B (2011). "Review and Hypothesis: Does Graves' Disease Develop in Non-Human Great Apes?". Immunology, Autoimmunity, and Graves' Ophthalmopathy. Thyroid. 21 (12). Mary Ann Liebert, Inc. (American Thyroid Association (ATA)): 1359–1366. doi:10.1089/thy.2011.0209. PMC 3229821. PMID 22066476.
- ^ a b McDermott MT (2019), McDermott MT (ed.), "Rogue Practitioners and Practices", Management of Patients with Pseudo-Endocrine Disorders: A Case-Based Pocket Guide, Cham: Springer International Publishing, pp. 23–30, doi:10.1007/978-3-030-22720-3_3, ISBN 978-3-030-22720-3, retrieved 4 December 2024
