Copper(I) chloride

| |

| |
| Names | |
|---|---|
| IUPAC name
Copper(I) chloride
| |
| Other names
Cuprous chloride
| |
| Identifiers | |
3D model (JSmol)
|
|
| 8127933 | |
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.028.948 |
| EC Number |
|
| 13676 | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| CuCl | |
| Molar mass | 98.999 g/mol[1] |
| Appearance | white powder, slightly green from oxidized impurities |
| Density | 4.14 g/cm3[1] |
| Melting point | 423 °C (793 °F; 696 K)[1] |
| Boiling point | 1,490 °C (2,710 °F; 1,760 K) (decomposes)[1] |
| 0.047 g/L (20 °C)[1] | |
Solubility product (Ksp)
|
1.72×10−7 |
| Solubility | insoluble in ethanol, acetone;[1] soluble in concentrated HCl, NH4OH |
| Band gap | 3.25 eV (300 K, direct)[2] |
| -40.0·10−6 cm3/mol[3] | |
Refractive index (nD)
|
1.930[4] |
| Structure | |
| Zincblende, cF20 | |
| F43m, No. 216[5] | |
a = 0.54202 nm
| |
Lattice volume (V)
|
0.1592 nm3 |
Formula units (Z)
|
4 |
| Hazards | |
| GHS labelling: | |
 
| |
| Warning | |
| H302, H410 | |
| P264, P270, P273, P301+P312, P330, P391, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
140 mg/kg |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 1 mg/m3 (as Cu)[6] |
REL (Recommended)
|
TWA 1 mg/m3 (as Cu)[6] |
IDLH (Immediate danger)
|
TWA 100 mg/m3 (as Cu)[6] |
| Safety data sheet (SDS) | JT Baker |
| Related compounds | |
Other anions
|
Copper(I) fluoride Copper(I) bromide Copper(I) iodide |
Other cations
|
Silver(I) chloride Gold(I) chloride |
Related compounds
|
Copper(II) chloride |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Copper(I) chloride, commonly called cuprous chloride, is the lower chloride of copper, with the formula CuCl. The substance is a white solid sparingly soluble in water, but very soluble in concentrated hydrochloric acid. Impure samples appear green due to the presence of copper(II) chloride (CuCl2).
History
[edit]Copper(I) chloride was first prepared by Robert Boyle in the mid-seventeenth century from mercury(II) chloride ("Venetian sublimate") and copper metal:[7]
- HgCl2 + 2 Cu → 2 CuCl + Hg
In 1799, J.L. Proust characterized the two different chlorides of copper. He prepared CuCl by heating CuCl2 at red heat in the absence of air, causing it to lose half of its combined chlorine followed by removing residual CuCl2 by washing with water.[8]
An acidic solution of CuCl was formerly used to analyze carbon monoxide content in gases, for example in Hempel's gas apparatus where the CuCl absorbs the carbon monoxide.[9] This application was significant during the nineteenth and early twentieth centuries when coal gas was widely used for heating and lighting.[10]
Synthesis
[edit]Copper(I) chloride is produced industrially by the direct combination of copper metal and chlorine at 450–900 °C:[11][12]
- 2 Cu + Cl2 → 2 CuCl
Copper(I) chloride can also be prepared by reducing copper(II) chloride with sulfur dioxide, or with ascorbic acid (vitamin C) that acts as a reducing sugar:[13][14]
- 2 CuCl2 + SO2 + 2 H2O → 2 CuCl + H2SO4 + 2 HCl
- 2 CuCl2 + C6H8O6 → 2CuCl + 2HCl + C6H6O6
Many other reducing agents can be used.[12]
-
White copper(I) chloride crystals on copper wire
-
Copper(I) chloride partially oxidized in air
Properties
[edit]Copper(I) chloride has the cubic zincblende crystal structure at ambient conditions. Upon heating to 408 °C the structure changes to hexagonal. Several other crystalline forms of CuCl appear at high pressures (several GPa).[5]
Copper(I) chloride is a Lewis acid. It is classified as soft according to the hard-soft acid-base concept. Thus, it forms a series of complexes with soft Lewis bases such as triphenylphosphine:
- CuCl + 1 P(C6H5)3 → 1/4 {CuCl[P(C6H5)3]}4
- CuCl + 2 P(C6H5)3 → CuCl[P(C6H5)3)]2
- CuCl + 3 P(C6H5)3 → CuCl[P(C6H5)3)]3
CuCl also forms complexes with halides. For example H3O+ CuCl2− forms in concentrated hydrochloric acid.[15] Chloride is displaced by CN− and S2O32−.[12]
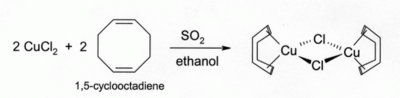
Solutions of CuCl in HCl absorb carbon monoxide to form colourless complexes such as the chloride-bridged dimer [CuCl(CO)]2. The same hydrochloric acid solutions also react with acetylene gas to form [CuCl(C2H2)]. Ammoniacal solutions of CuCl react with acetylenes to form the explosive copper(I) acetylide, Cu2C2. Alkene complexes of CuCl can be prepared by reduction of CuCl2 by sulfur dioxide in the presence of the alkene in alcohol solution. Complexes with dienes such as 1,5-cyclooctadiene are particularly stable:[16]
Upon contact with water, copper(I) chloride slowly undergoes disproportionation:[17]
- 2 CuCl → Cu + CuCl2
In part for this reason, samples in air assume a green coloration.[18]
Uses
[edit]The main use of copper(I) chloride is as a precursor to the fungicide copper oxychloride. For this purpose aqueous copper(I) chloride is generated by comproportionation and then air-oxidized:[12]
- Cu + CuCl2 → 2 CuCl
- 4 CuCl + O2 + 2 H2O → Cu3Cl2(OH)4 + CuCl2
Copper(I) chloride catalyzes a variety of organic reactions, as discussed above. Its affinity for carbon monoxide in the presence of aluminium chloride is exploited in the COPureSM process.[19]
In organic synthesis
[edit]CuCl is used as a co-catalyst with carbon monoxide, aluminium chloride, and hydrogen chloride in the Gatterman-Koch reaction to form benzaldehydes.[20]
In the Sandmeyer reaction, the treatment of an arenediazonium salt with CuCl leads to an aryl chloride. For example:[21][22]
The reaction has wide scope and usually gives good yields.[22]
Early investigators observed that copper(I) halides catalyse 1,4-addition of Grignard reagents to alpha,beta-unsaturated ketones[23] led to the development of organocuprate reagents that are widely used today in organic synthesis:[24]
This finding led to the development of organocopper chemistry. For example, CuCl reacts with methyllithium (CH3Li) to form "Gilman reagents" such as (CH3)2CuLi, which find use in organic synthesis. Grignard reagents form similar organocopper compounds. Although other copper(I) compounds such as copper(I) iodide are now more often used for these types of reactions, copper(I) chloride is still recommended in some cases:[25]
Cuprous chloride also catalyzes the dimerization of acetylene to vinylacetylene, once used as a precursor to various polymers such a neoprene.[26]
Niche uses
[edit]CuCl is used as a catalyst in atom transfer radical polymerization (ATRP). It is also used in pyrotechnics as a blue/green coloring agent. In a flame test, copper chlorides, like all copper compounds, emit green-blue.[27]
Natural occurrence
[edit]Natural form of CuCl is the rare mineral nantokite.[28][29]
See also
[edit]References
[edit]- ^ a b c d e f Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.61. ISBN 1-4398-5511-0.
- ^ Garro, Núria; Cantarero, Andrés; Cardona, Manuel; Ruf, Tobias; Göbel, Andreas; Lin, Chengtian; Reimann, Klaus; Rübenacke, Stefan; Steube, Markus (1996). "Electron-phonon interaction at the direct gap of the copper halides". Solid State Communications. 98 (1): 27–30. Bibcode:1996SSCom..98...27G. doi:10.1016/0038-1098(96)00020-8.
- ^ Haynes, William M., ed. (2011). CRC Handbook of Chemistry and Physics (92nd ed.). Boca Raton, FL: CRC Press. p. 4.132. ISBN 1-4398-5511-0.
- ^ Patnaik, Pradyot (2002) Handbook of Inorganic Chemicals. McGraw-Hill, ISBN 0-07-049439-8
- ^ a b Hull, S.; Keen, D. A. (1994). "High-pressure polymorphism of the copper(I) halides: A neutron-diffraction study to ~10 GPa". Physical Review B. 50 (9): 5868–5885. Bibcode:1994PhRvB..50.5868H. doi:10.1103/PhysRevB.50.5868. PMID 9976955.
- ^ a b c NIOSH Pocket Guide to Chemical Hazards. "#0150". National Institute for Occupational Safety and Health (NIOSH).
- ^ Boyle, Robert (1666). Considerations and experiments about the origin of forms and qualities. Oxford. pp. 286–288.
- ^ Proust, J. L. (1799). "Recherches sur le Cuivre". Ann. Chim. Phys. 32: 26–54.
- ^ Martin, Geoffrey (1922). Industrial and Manufacturing Chemistry (Part 1, Organic ed.). London: Crosby Lockwood. p. 408.
- ^ Lewes, Vivian H. (1891). "The Analysis of Illuminationg Gases". Journal of the Society of Chemical Industry. 10: 407–413.
- ^ Richardson, H. W. (2003). "Copper Compounds". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.0315161618090308.a01.pub2. ISBN 0471238961.
- ^ a b c d Zhang, J.; Richardson, H. W. (2016). "Copper Compounds". Ullmann's Encyclopedia of Industrial Chemistry. pp. 1–31. doi:10.1002/14356007.a07_567.pub2. ISBN 978-3-527-30673-2.
- ^ Glemser, O.; Sauer, H. (1963). "Copper(I) Chloride". In Brauer, G. (ed.). Handbook of Preparative Inorganic Chemistry. Vol. 1 (2nd ed.). New York: Academic Press. p. 1005.
- ^ Tuğba Akbıyık; İnci Sönmezoğlu; Kubilay Güçlü; İzzet Tor; Reşat Apak (2012). "Protection of Ascorbic Acid from Copper(II)−Catalyzed Oxidative Degradation in the Presence of Fruit Acids: Citric, Oxalic, Tartaric, Malic, Malonic, and Fumaric Acids". International Journal of Food Properties. 15 (2): 398–411. doi:10.1080/10942912.2010.487630. S2CID 85408826.
- ^ J. J. Fritz (1980). "Chloride complexes of copper(I) chloride in aqueous solution". J. Phys. Chem. 84 (18): 2241–2246. doi:10.1021/j100455a006.
- ^ Nicholls, D. (1973) Complexes and First-Row Transition Elements, Macmillan Press, London.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 1185. ISBN 978-0-08-037941-8.
- ^ Pastor, Antonio C. (1986) U.S. patent 4,582,579 "Method of preparing cupric ion free cuprous chloride" Section 2, lines 4–41.
- ^ Xiaozhou Ma; Jelco Albertsma; Dieke Gabriels; Rens Horst; Sevgi Polat; Casper Snoeks; Freek Kapteijn; Hüseyin Burak Eral; David A. Vermaas; Bastian Mei; Sissi de Beer; Monique Ann van der Veen (2023). "Carbon monoxide separation: past, present and future". Chemical Society Reviews. 52 (11): 3741–3777. doi:10.1039/D3CS00147D. PMC 10243283. PMID 37083229.
- ^ Dilke, M. H.; Eley, D. D. (1949). "550. The Gattermann–Koch reaction. Part II. Reaction kinetics". J. Chem. Soc.: 2613–2620. doi:10.1039/JR9490002613. ISSN 0368-1769.
- ^ Wade, L. G. (2003) Organic Chemistry, 5th ed., Prentice Hall, Upper Saddle River, New Jersey, p. 871. ISBN 013033832X.
- ^ a b March, J. (1992) Advanced Organic Chemistry, 4th ed., Wiley, New York. p. 723. ISBN 978-0-470-46259-1
- ^ Kharasch, M. S.; Tawney, P. O. (1941). "Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide". J. Am. Chem. Soc. 63 (9): 2308. doi:10.1021/ja01854a005.
- ^ Jasrzebski, J. T. B. H.; van Koten, G. (2002) Modern Organocopper Chemistry, N. Krause (ed.). Wiley-VCH, Weinheim, Germany. p. 1. doi:10.1002/3527600086.ch1 ISBN 9783527600083.
- ^ Bertz, S. H.; Fairchild, E. H. (1999) Handbook of Reagents for Organic Synthesis, Volume 1: Reagents, Auxiliaries and Catalysts for C-C Bond Formation, R. M. Coates, S. E. Denmark (eds.). Wiley, New York. pp. 220–3. ISBN 978-0-471-97924-1.
- ^ Trotuş, Ioan-Teodor; Zimmermann, Tobias; Schüth, Ferdi (2014). "Catalytic Reactions of Acetylene: A Feedstock for the Chemical Industry Revisited". Chemical Reviews. 114 (3): 1761–1782. doi:10.1021/cr400357r. PMID 24228942.
- ^ Barrow, R F; Caldin, E F (1949-01-01). "Some Spectroscopic Observations on Pyrotechnic Flames". Proceedings of the Physical Society. Section B. 62 (1): 32–39. doi:10.1088/0370-1301/62/1/305. ISSN 0370-1301.
- ^ "Nantokite".
- ^ "List of Minerals". 21 March 2011.
External links
[edit]- National Pollutant Inventory – Copper and compounds fact sheet
- The COPureSM Process for purifying CO utilizing a copper chloride complex